Abstract
Introduction: VEXAS (vacuoles, E1 enzyme, X-linked, autoinflammatory, somatic) syndrome is characterized by adult-onset inflammatory manifestations with predisposition to myelodysplastic syndromes (MDS). Given the higher median age at onset and prevalent inflammation, approximately 60% of patient have concurrent clonal hematopoiesis (CH) involving epigenetic regulators (epi), DNMT3A and TET2, with a negative impact on survival (see accompanying ASH abstract). DNMT inhibitors, considered standard of care for MDS, have anecdotally been used in VEXAS with mixed responses. However, to date, the epigenetic landscape in VEXAS has not been defined. We carried out this study to assess changes in DNA methylation in VEXAS syndrome.
Methods: After IRB approval, Mayo Clinic and NIH patients with a confirmed diagnosis of VEXAS syndrome were included. DNA was extracted from blood mononuclear cells and error-corrected DNA sequencing was performed to define the CH landscape (see accompanying ASH abstract). DNA Methylation changes were identified using the Illumina Infinium MethylationEPIC BeadChip. Conventional bisulfite-treatment was completed using the TrueMethyl oxBS Module (Tecan Genomics, Männedorf, Switzerland). Briefly, DNA was purified using magnetic beads, denatured, and underwent bisulfite conversion followed by desulfonation and purification. The TrueMethyl converted DNA samples were then eluted and processed through the MethylationEPIC BeadChip array protocol. Subset-quantile Within Array Normalization (SWAN) was performed via the R package "minfi". The resultant β-values were used to identify changes in DNA methylation (Δβ) between groups. Whole transcriptome RNAseq is currently pending on a subset of patients.
Results: Fifty male patients with VEXAS, without a diagnosis of MDS were included in this study; median age 70.5 years (range: 48 - 89). "Typical non-UBA1 CH mutations" were seen in 30 (60%) patients, predominantly involving DNMT3A and TET2 (DNMT3A n=10, TET2 n=8, DNMT3A/TET2 n=6; 48%). Subclonal CH mutations in PPM1D (N=2), KRAS and TP53 (n=1 each) were seen in 4 patients with variant allele fractions (VAF) <2%. The median VAF for DNMT3A was 28% and for TET2 was 2%, respectively. Thirty (60%) patients had UBA1Met41T substitutions, 14 (28%) had Met41V and 3 (8%) each had Met41L substitutions and splice site mutations, respectively.
DNA methylation was first compared to 8 healthy males, with a median age of 59 years (Age range: 42 - 61). VEXAS patients had >2,800 differentially methylated CpGs, with 77% of the CpGs being hypermethylated compared to the healthy controls. Additional age matched controls are currently being assessed to eliminate the age bias in this analysis.
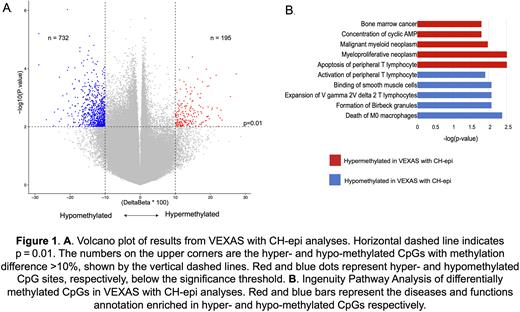
We then compared methylation changes in VEXAS patients with DNMT3A and/or TET2 CH with those without (n=24 vs n=36, median ages not significantly different). VEXAS patients with CH-epi mutations had >900 differentially methylated CpGs when compared to VEXAS patients without CH-epi. Majority of these differentially methylated CpGs were hypomethylated (79%) in the VEXAS patients with CH-epi (Figure 1A). Ingenuity pathway analysis demonstrated that the hypomethylated loci resulted in transcriptional activation of the following pathways/genes; apoptosis of peripheral T lymphocytes (p=0.003, EZR and MAPK8) and multiple convergent myeloid neoplasm pathways (p<0.003, WT1 and MPL). Hypermethylated loci in CH-epi resulted in transcriptional repression of cell death of M0 macrophages (p=0.0048, IRF8), expansion of gamma/delta T cells (p=0.008, BTNL9), activation of peripheral T lymphocytes (p=0.025, MFN2) and aggregation of monocytes (p=0.002, BMP7) (Figure 1B). RNA sequencing confirming these changes is pending in 14 cases. We did not assess differences between DNMT3A and TET2 mutant groups given the smaller samples size and the fact that the median TET2 mutant VAF was 2%.
Conclusions: We demonstrate DNA methylation changes in a large cohort of VEXAS patients, exploring the negative impact of CH-epi on survival. We show that VEXAS patients with CH-epi are enriched in hypomethylated CpG sites in comparison to those without CH-epi. Pathway analysis suggest roles for WT1, a critical transcription factor and oncogene and MPL, the platelet thrombopoietin receptor, in disease biology and survival. Integration with RNAseq and recruitment of additional patients and controls is ongoing.
Disclosures
Mangaonkar:Bristol Myers Squibb: Research Funding. Warrington:Eli Lilly: Other: Clinical Trial Support; GSK: Other: Clinical Trial Support; Kiniksa: Other: Clinical Trial Support; Chemocentryx: Consultancy. Patnaik:Stem Line Pharmaceuticals: Research Funding; Kura Oncology: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal